Dr. Koichi Suzuki
Professor, Department of Clinical Laboratory Science
Faculty of Medical Technology, Teikyo University
Professor Suzuki is a basic scientist in the field of leprosy and other neglected tropical diseases. Before moving to Teikyo University, he served for 15 years as chief of the Laboratory of Molecular Diagnostics at Japan’s National Institute of Infectious Diseases.
https://plaza.umin.ac.jp/suzuki-lab/
At present, definitive leprosy diagnosis relies on laboratory-based PCR testing involving expensive equipment, refrigeration, and highly trained technicians. Testing for drug resistance requires an additional scientific instrument called a DNA sequencer. Access to these resources is limited in many parts of the world, and so many persons affected by leprosy have no choice but to accept inadequate diagnosis and inappropriate treatment.
A research team that I lead along with Assistant Professor Kei Mikita of Keio University and Senior Researcher Hiroyuki Miyamura of the National Institute of Advanced Industrial Science and Technology is trying to change this situation. With support from the Sasakawa Health Foundation and others, we are developing a method that can be used for diagnostic testing at point of care in resource-limited settings. We call it the CRADAR-i method.
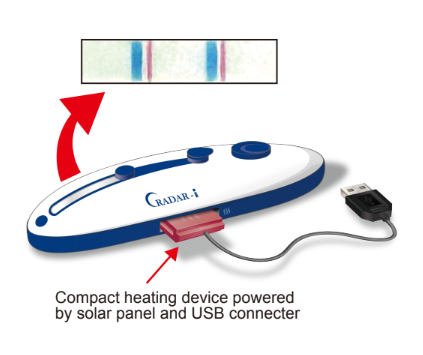
With this method, we can provide all the necessary reagents for DNA amplification and detection within a small device that does not require refrigeration. Device kits are easily portable and can be stored for up to one year at ambient temperature in tropical environments. Operation is simple: Just drop in a sample and press a few buttons. Results appear within one hour in the form of a blue line visible to the naked eye. Compared to conventional PCR and DNA sequencing methods, the CRADAR-i method reduces cost of detection while maintaining sensitivity.
Testing for M. leprae and rifampicin-resistant strains
We can configure a CRADAR-i device so that it detects Mycobacterium leprae as well as mutations that confer resistance to the drug rifampicin. Instead of diagnosing leprosy based on patient history and physical examination only, or waiting days for results from a laboratory, healthcare providers will be able to test immediately for M. leprae and make a same-day definitive diagnosis. They will also be able to make decisions about antimicrobial therapy and post-exposure prophylaxis (PEP) based on evidence of susceptibility. This latter point is especially important in the context of the World Health Organization’s recommendation to administer single-dose rifampicin post-exposure prophylaxis (SDR-PEP) to close contacts to prevent transmission. Rifampicin susceptibility is a minimum requirement for the success of this strategy.
Working to make the devices available
In 2022, we shared a report about the CRADAR-i method at two events in India: the International Leprosy Congress in Hyderabad (Nov. 8–11) and the “Workshop on strengthening laboratory testing procedures for antimicrobial resistance (AMR) surveillance in leprosy” organized by WHO in Karigiri (Nov. 14–15). As of June 2024, we have confirmed that CRADAR-i works well in the laboratory. The next step is to recruit partners to mass produce device kits and make them available worldwide either free of charge or at very low cost.
The CRADAR-i method addresses major challenges affecting leprosy diagnosis, treatment, and prevention of transmission. We expect that field-deployable devices using the CRADAR-i method will play a crucial role in helping WHO achieve its zero leprosy goals.